- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Cancer Biotech Day One Biopharma Expands to ADCs, Paying $55M to Nab a Phase 1-Ready Drug | 1 | MedCity News | ||
| Di | Day One Biopharmaceuticals, Inc.: Day One Expands Pipeline with Potential First-in-Class Clinical-Stage Antibody Drug Conjugate (ADC) Targeting PTK7 in Solid Tumors for Adult and Pediatric Cancers | 26 | GlobeNewswire (Europe) | Day One receives exclusive license for development and commercialization of MTX-13 (DAY301), which received IND clearance by the FDA in April 2024 Targets PTK7, highly expressed in broad range of... ► Artikel lesen | |
| 12.06. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.06. | XOMA Corporation: XOMA Receives $8.1 Million Milestone Related to Day One Biopharmaceuticals' Sale of its Priority Review Voucher | 68 | GlobeNewswire (Europe) | EMERYVILLE, Calif., June 12, 2024 (GLOBE NEWSWIRE) -- XOMA Corporation (NASDAQ: XOMA), the biotech royalty aggregator, announced today it has received an $8.1 million milestone payment from Viracta... ► Artikel lesen | |
| 30.05. | Day One Biopharmaceuticals announces sale of Priority Review Voucher for $108M | 2 | Seeking Alpha | ||
| 30.05. | Day One Biopharmaceuticals, Inc.: Day One Announces Sale of Priority Review Voucher for $108 Million | 1 | GlobeNewswire (USA) | ||
| 24.05. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 07.05. | Day One Biopharmaceuticals GAAP EPS of -$0.72 misses by $0.06 | 3 | Seeking Alpha | ||
| 06.05. | Day One Biopharmaceuticals, Inc.: Day One Reports First Quarter 2024 Financial Results and Corporate Progress | 171 | GlobeNewswire (Europe) | OJEMDA (tovorafenib) launch underway following U.S. FDA accelerated approval for relapsed or refractory BRAF-altered Pediatric Low-Grade Glioma (pLGG) First prescriptions received in the U.S. BRISBANE... ► Artikel lesen | |
| 06.05. | Day One Biopharmaceuticals, Inc. - 10-Q, Quarterly Report | 1 | SEC Filings | ||
| 06.05. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 24.04. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 23.04. | Day One Biopharmaceuticals, Inc.: Day One's OJEMDA (tovorafenib) Receives US FDA Accelerated Approval for Relapsed or Refractory BRAF-altered Pediatric Low-Grade Glioma (pLGG), the Most Common Form of Childhood Brain Tumor | 58 | GlobeNewswire (Europe) | First and only FDA-approved type II RAF inhibitor for patients with relapsed or refractory pLGG harboring a BRAF fusion or rearrangement, or BRAF V600 mutation RAPNO LGG overall response rate... ► Artikel lesen | |
| 23.04. | Day One Biopharma gains on FDA's accelerated nod for brain tumor treatment | 3 | Reuters | ||
| 07.03. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 26.02. | Day One Biopharmaceuticals reports Q4 results | 1 | Seeking Alpha | ||
| 26.02. | Day One Biopharmaceuticals, Inc.: Day One Reports Fourth Quarter and Full Year 2023 Financial Results and Corporate Progress | 190 | GlobeNewswire (Europe) | PDUFA target action date for tovorafenib NDA in relapsed or progressive pLGG remains set for April 30, 2024 Phase 2 FIREFLY-1 tovorafenib registrational data published in Nature Medicine Ended 2023... ► Artikel lesen | |
| 17.01. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 08.01. | Day One Biopharmaceuticals, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 04.01. | Day One Biopharmaceuticals, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 1 | SEC Filings |
Seite:
1 2 Weiter >>
24 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| EPIGENOMICS | 1,010 | -25,46 % | EQS-News: Epigenomics AG: Ausscheiden des Vorstands Jens Ravens zum 30. April 2024 | EQS-News: Epigenomics AG
/ Schlagwort(e): Personalie
Epigenomics AG: Ausscheiden des Vorstands Jens Ravens zum 30. April 2024
30.04.2024 / 15:00 CET/CEST
Für den Inhalt... ► Artikel lesen | |
| DEFENCE THERAPEUTICS | 0,612 | +0,66 % | EQS-News: Defence Therapeutics Inc.: Der wissenschaftliche Leiter von Defence, Dr. Moutih Rafei, spricht auf Money Talk Radio mit Ellis Martin über die Accum-Platform zur Krebsbekämpfung | EQS-News: Defence Therapeutics Inc.
/ Schlagwort(e): Sonstiges
DER WISSENSCHAFTLICHE LEITER VON DEFENCE, DR. MOUTIH RAFEI, SPRICHT AUF MONEY TALK RADIO MIT... ► Artikel lesen | |
| GINKGO BIOWORKS | 0,392 | -0,51 % | Ginkgo Bioworks Holdings, Inc. - 8-K, Current Report | ||
| BEAM THERAPEUTICS | 23,170 | 0,00 % | Beam Therapeutics Reports Data Highlighting Optimized Manufacturing Process for BEAM-101, an Investigational Base Editing Therapeutic for Sickle Cell Disease | MADRID, June 14, 2024 (GLOBE NEWSWIRE) -- Beam Therapeutics Inc. (Nasdaq: BEAM), a biotechnology company developing precision genetic medicines through base editing, today reported data highlighting... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 9,700 | +3,52 % | Recursion Pharmaceuticals: Recursion Announces Completion of NVIDIA-Powered BioHive-2, the Largest Supercomputer in Pharmaceutical Industry | SALT LAKE CITY, May 13, 2024 (GLOBE NEWSWIRE) -- Recursion (NASDAQ: RXRX), a leading clinical stage TechBio company decoding biology to industrialize drug discovery, today announced the completion... ► Artikel lesen | |
| ONCOLYTICS BIOTECH | 0,945 | 0,00 % | Oncolytics Biotech® Inc.: Oncolytics Biotech® ASCO Abstracts Highlight Pelareorep's Potential in Pancreatic Cancer and Immunotherapeutic Mechanism of Action | Trial-in-progress abstract highlights new cohort that could expand the company's pancreatic cancer program
Pelareorep's ability to expand TILs highlights its immunotherapeutic mechanism... ► Artikel lesen | |
| ASSERTIO | 1,095 | +4,29 % | Assertio Holdings And 2 Other Penny Stocks Insiders Are Buying | ||
| ARCTURUS THERAPEUTICS | 25,960 | 0,00 % | Arcturus Therapeutics Holdings Inc. - 8-K, Current Report | ||
| VIR BIOTECHNOLOGY | 8,950 | -2,51 % | Vir Biotechnology, Inc.: Multiple Abstracts Highlighting Vir Biotechnology's Latest Hepatitis Delta & B Data Accepted for Presentation at the EASL Congress 2024 | - Company to present important new data from the Phase 2 SOLSTICE chronic hepatitis delta trial - - Conference call scheduled for June 5, 2024, at 6:00 a.m. ET 12:00 p.m. CEST -
Vir Biotechnology... ► Artikel lesen | |
| SIGA TECHNOLOGIES | 6,330 | 0,00 % | SIGA Technologies Inc.: SIGA Announces Agreement to Sell TPOXX® to ASEAN Member States | ||
| MERRIMACK PHARMACEUTICALS | 15,130 | 0,00 % | Merrimack Pharmaceuticals, Inc. Announces Stockholder Approval of Plan of Dissolution And Cash Liquidating Dividend Amount of $15.10 Per Share of Common Stock | CAMBRIDGE, Mass.--(BUSINESS WIRE)--Merrimack Pharmaceuticals, Inc. (Nasdaq: MACK) ("Merrimack" or the "Company") announced that the stockholders at a Special Meeting held today overwhelmingly approved... ► Artikel lesen | |
| VERVE THERAPEUTICS | 5,100 | 0,00 % | Verve Therapeutics Announces Inducement Grants under Nasdaq Listing Rule 5635(c)(4) | BOSTON, May 31, 2024 (GLOBE NEWSWIRE) -- Verve Therapeutics, a clinical-stage biotechnology company pioneering a new approach to the care of cardiovascular disease with single-course gene editing... ► Artikel lesen | |
| ARS PHARMACEUTICALS | 8,100 | 0,00 % | ARS Pharmaceuticals, Inc.: ARS Pharmaceuticals Submits Response for neffy (epinephrine nasal spray) Marketing Authorization Application to EMA's CHMP and Enters License Agreement with CSL Seqirus for Commercialization of neffy in Australia and New Zealand | CHMP opinion on neffy Marketing Authorization Application anticipated in the second quarter of 2024 Response addresses all issues previously identified by CHMP, and includes results from a repeat... ► Artikel lesen | |
| ADMA BIOLOGICS | 10,620 | 0,00 % | ADMA Biologics, Inc.: ADMA Biologics Announces FDA Approvals of Extended Room Temperature Storage Conditions for ASCENIV & BIVIGAM | FDA Approval Provides for Room Temperature (25°C) Storage Conditions for up to 4 Weeks at Any Point During the 36-Month Approved Shelf Life Extends the Prior Room Temperature Storage Allowance for... ► Artikel lesen | |
| BIO-PATH | 2,270 | 0,00 % | Why Is Bio-Path (BPTH) Stock Up 20% Today? |
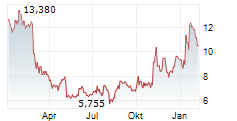
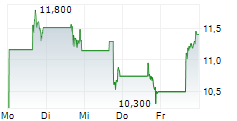
Das Unternehmen DAY ONE BIOPHARMACEUTICALS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 13,390 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und